Hemophilia B is an inherited, X-linked, recessive disorder that results from deficiency of the functional plasma coagulation factor IX (hFIX). People with hemophilia B bleed longer than other people. Bleeds can occur into joints, soft tissues and mucous membranes. Hemophilia occurs in approximately 1 in 25,000 males worldwide. The main medication to treat hemophilia B is concentrated FIX product. There is currently no cure for hemophilia.
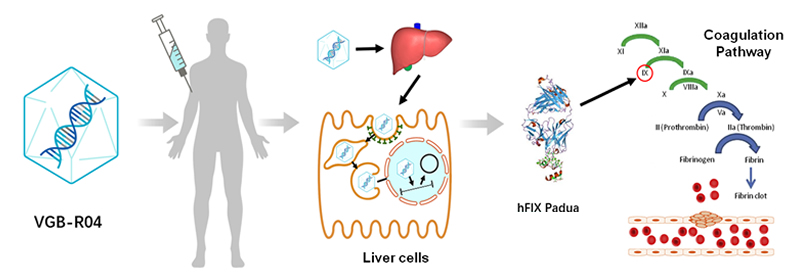
VGB-R04 treatment strategy: replacement of the defective factor IX gene by ssAAV8 delivery of a functional factor IX transgene via iv injection for hepatic expression and secretion.
VGB-R04 is a rAAV product containing a variant hFIX Padua expression cassette under the control of a liver specific promoter. The hFIX Padua variant is a natural mutant found in an HB family with increased specific activity. After intravenous injection of VGB-R04, the hFIX expression cassette will be delivered into the liver cells and become episomal to continuously express and secret hFIX Padua protein into the circulation to replace the missing hFIX protein to normalize the blood clotting function in hemophilia B patients. Due to its expected long-term expression of hFIX, this product will be a one-time i.v. administration to provide long-term treatment for the hemophilia B patients.
Vitalgen has initiated and carried out a clinical study and is currently recruiting.
Learn More
VGB-R04 was granted a tacit approval for clinical trials by NMPA on April, 2022. VGB-R04 was also granted ODD by FDA on December, 2021, which is the first in vivo gene therapy product developed in China for hemophilia B that has received ODD.