On November 1, 2022, the clinical trial (IND) application for VGR-R01 injection developed independently by Shanghai Vitalgen BioPharma Co., Ltd. (hereinafter referred to as “Vitalgen”) received the implied permission from the Center for Drug Evaluation (CDE) National Medical Products Administration. VGR-R01 is a gene replacement therapy drug for patients with Bietti crystalline dystrophy (BCD) caused by CYP4V2 gene mutation, and is the second clinically approved gene therapy product from Vitalgen after the approval of VGB-R04 injection in July this year.
May all diseases have a chance of curing
The Report of the 20th Party Congress proposes to put the protection of people's health in the strategic position of priority development. The article “All for the People's Health - Medical and Health Workers Strive to Safeguard People's Health in an All-round Way in Full Cycle” written by Xinhua News Agency states that “to building a healthy China, no one should be left behind”, directly facing the challenge of how to bring the light of hope to patients with rare diseases nationwide. For rare diseases like BCD which have received little attention, Dr. Xiaoping Zhao, CEO of Vitalgen, said, “We have a sense of mission to invent drugs for patients with rare diseases in China. VGR-R01 is the world's first BCD therapeutic drug approved for clinical trials, giving hope to patients with incurable BCD in China and around the world. We hope to transform gene delivery and gene editing technologies into gene therapies for curing diseases and pains through our unremitting efforts to help the construction of an all-round healthy China, and also let the world feel the warmth and goodwill of biotechnology.”
Figure 1. CDE public information
About BCD
Bietti crystalline dystrophy (BCD), also known as Bietti crystalline retinopathy, is an autosomal recessive, progressive retinal degenerative disease. The pathogenic gene for BCD is CYP4V2 located at 4q35, which encodes a protease involved in lipid metabolism. BCD is found worldwide, but more prevalent in Chinese, Japanese and Korean populations. Most patients with BCD develop symptoms such as night blindness and decreased visual acuity in their 20s and 40s, and develop legal blindness in their 50s and 60s. BCD can be diagnosed based on clinical characteristics and genetic testing to identify the biallelic pathogenic variation in CYP4V2, but there is still no effective clinical treatment option available.
About VGR-R01
VGR-R01 is a gene therapy product for patients with BCD caused by CYP4V2 gene mutation. CYP4V2 protein is a member of the P450 enzyme family. It is highly expressed in retinal pigment epithelium (RPE), with fatty acid hydroxylase activity, and is related to lipid metabolism. Mechanism of action of VGR-R01 is gene replacement.
After VGR-R01 is administered via subretinal injection, AAV capsid protein mediates transduction of RPE cells, delivering the VGR-R01 gene expression cassette to the nucleus. The VGR-R01 expression cassette exists as free DNA and expresses CYP4V2 protein in RPE cells to reconstitute the fatty acid hydroxylase activity of the cells. VGR-R01 aims to prevent or improve the structural and/or functional damage of RPE cells, photoreceptor cells and choroid by correcting the fatty acid metabolism disorder in the retina of patients, so as to correct visual impairment, protect residual visual function, or delay vision deterioration.
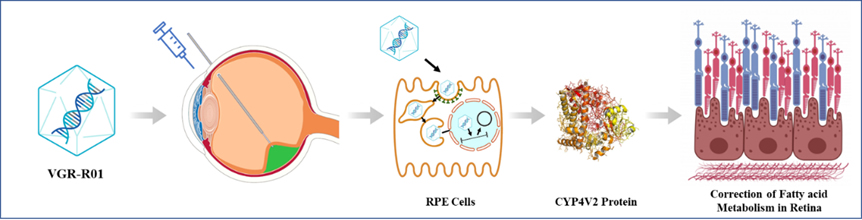
Figure 2. Mechanism of action of VGR-R01
Early clinical study of VGR-R01 “An Early Clinical Study of VGR-R01 in the Treatment of Bietti Crystalline Dystrophy (BCD)” (NCT05399069) is ongoing at Beijing Tongren Hospital Affiliated to Capital Medical University. Now dosing of some subjects has been completed, and preliminary results suggest that VGR-R01 has a favorable safety profile and tends to improve the visual function.
Principal Investigator
Professor Wei Wenbin, Professor Zhao Xiuli
Contact
Mr. Zhang 13552757069, Dr. Wang (WeChat 13683178767)
Major inclusion criteria
1. Male or female with age ≥ 18 years old and < 80 years old.
2. Patients clinically diagnosed with BCD, with molecular diagnosis confirming CYP4V2 biallelic mutation;
3. Patients with best corrected visual acuity of the target eye ≤0.1;
4. Patients who must agree to use reliable contraceptions until at least one year after VGR-R01 injection;
5. Patients without serious systemic diseases.


